
Yes the equation works, but it’s far too tedious and annoying! There are just too many steps and calculations. A triple bond of 6 electrons allows each atom to claim three.ĭo you dread running through this every time you finish a reaction? A double bond containing four electrons allows each atom to claim 2.
Now that you know WHAT we’re studying, let’s see HOW! – That’s where I can help you. If the atom loses negative electrons and therefore has as few as one extra POSITIVE protons in its nucleus, it will carry a net positive charge. If the atom has just one more negative electron, than protons, it will have a net negative charge. An ion is simply an atom or molecule that gained or lost electrons to get a net charge. Think back to general chemistry when you studied ion formation. The answers are yes, yes, yes, and more yes. Which specific atom is responsible for the overall charge?Īnd, what if you’re studying an uncharged molecule – Is it still possible for individual atoms to carry charge despite the net neutrality? Even the negative charge on the hydroxide oxygen is simple to understand.īut what if you have a much larger group of bound atoms with an overall net charge? For example, the negative nitrate or triple negative phosphate. Chloride obviously has a negative charge. This concept is simple enough for small ions. The sum of formal charges on any molecule or ion results in the net overall charge. So what exactly IS formal charge?įormal charge is the actual charge on an individual atom within a larger molecule or polyatomic ion. Formal charge helps you understand reaction patterns by showing you why a specific atom attacks, and why its ‘victim’ accepts the attack. Yet understanding the nature of Formal Charge is a critical component when it comes to mastering organic chemistry reactions and mechanisms.

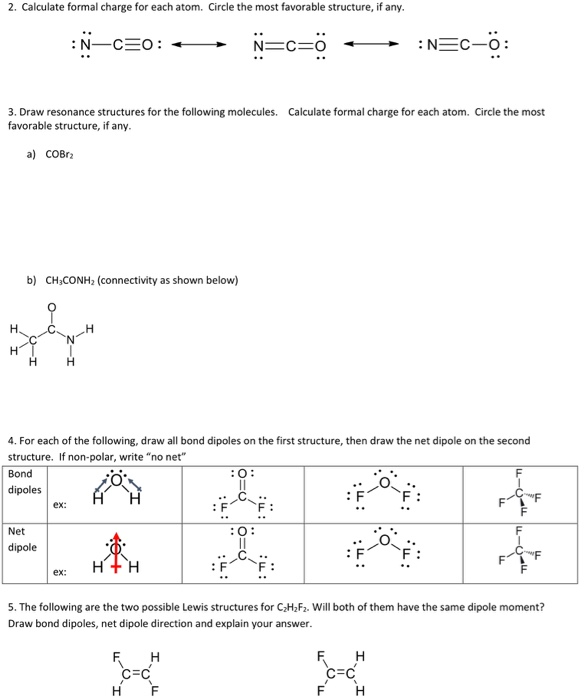
How can you afford that kind of time when working through a multi-step synthesis? This suggests that the seventh statement is true.Formal Charge is that pesky concept covered early in organic chemistry and somehow never seems to go away.īecause the equation in your textbook is long, confusing, and needlessly annoying. The formal charge on central N = 5 - 0 - 4 = +1 The formal charge on the central N atom can be calculated as follows-įormal charge = Number of valence electrons - Number of lone pair electrons - number of bonds This suggests that the sixth statement is false. However, the three resonance structures do not have the same stability and hence they are not equivalent resonance structures. There are three possible resonance structures for the N₂O molecule which can be represented as follows. The oxidation state of both N atoms in N₂O is +1. This suggests that there are two σ bonds and two π bonds in the molecule. Similarly, in the triple bond, one bond is σ and the two bonds are π. In the above structure, the N-O single bond is a σ bond. The hybridization of the central N atom is sp, which suggests that the molecule will be linear. The steric number value 2 suggests that the hybridization of central N is sp. Steric number = Number of atoms directly attached + number of lone pairs of electrons = 2+ 0 = 2 The steric number for the central N atom can be calculated as follows. The structure (without including the lone pairs) can be represented as follows. The number of lone pair electrons around the central N atom is 0. Moreover, there is a lone pair of electrons on the terminal N atom and three lone pairs of electrons on the O atom. There is a formal positive (+) charge on the central N atom and a formal negative (-) charge on the O atom. In the most stable Lewis structure of N₂O, the central nitrogen (N) atom forms a triple bond with the terminal N atom and a single bond with the O atom. The central N atom has a formal charge of +1. The two N atoms have the same oxidation state. The true statements can be listed as follows.


 0 kommentar(er)
0 kommentar(er)
